IVD Quality Control Market Overview
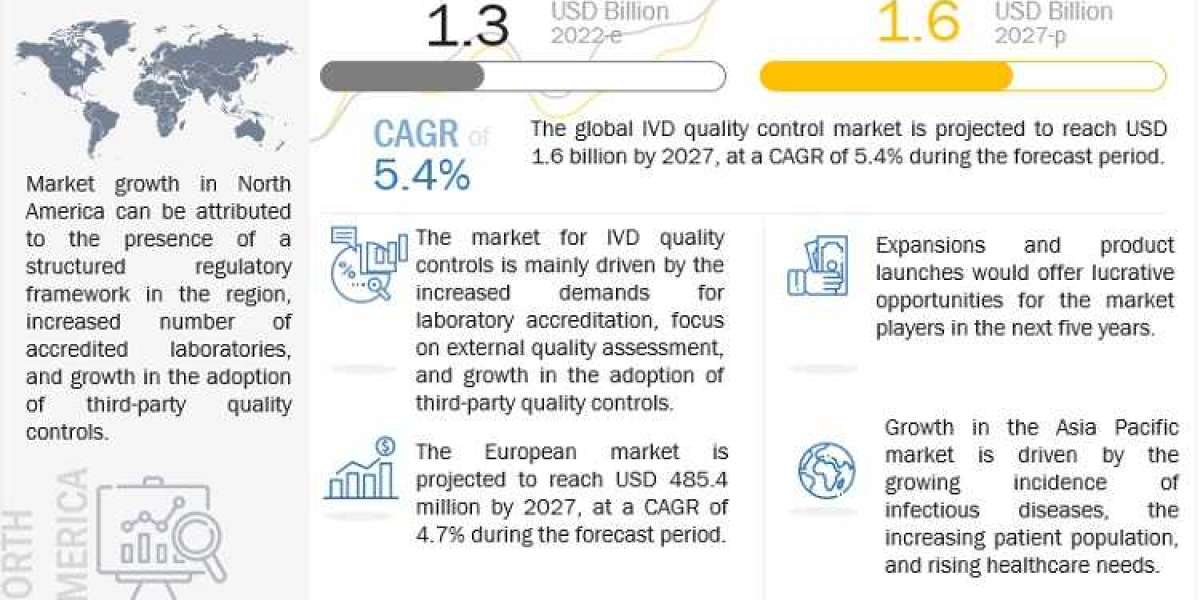
The In Vitro Diagnostics (IVD) quality control market has seen significant growth in recent years, driven by advancements in diagnostic technologies, increasing prevalence of chronic diseases, and a heightened focus on precision medicine. Quality control (QC) in IVD is crucial to ensure the accuracy, reliability, and performance of diagnostic tests, which are essential for patient care and clinical decision-making.
Request for FREE Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=198032582
Market Drivers
- Technological Advancements: Innovations in IVD technologies, such as next-generation sequencing (NGS), polymerase chain reaction (PCR), and immunoassays, have propelled the demand for robust quality control measures to maintain test accuracy.
- Rising Prevalence of Chronic Diseases: The increasing incidence of diseases such as cancer, diabetes, and cardiovascular diseases has led to a higher demand for diagnostic tests, thereby boosting the need for quality control in the IVD market.
- Regulatory Requirements: Stringent regulations by health authorities, such as the FDA and EMA, mandate rigorous quality control standards to ensure the safety and efficacy of diagnostic tests.
- Growth of Point-of-Care Testing: The shift towards point-of-care testing (POCT) has necessitated the development of QC measures that are easy to use and provide quick, accurate results.
Download an Illustrative overview: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=198032582
Market Segmentation
The IVD quality control market can be segmented based on product type, application, end-user, and region.
- By Product Type:
- Quality Control Products: Controls, calibrators, and reagents.
- Data Management Solutions: Software and systems for data analysis and quality control monitoring.
- By Application:
- Clinical Chemistry: Controls for tests measuring blood glucose, lipids, enzymes, and other chemical substances.
- Immunoassays: Controls for tests detecting antibodies, antigens, and proteins.
- Molecular Diagnostics: Controls for nucleic acid tests, including PCR and NGS.
- Microbiology: Controls for tests identifying bacterial, viral, and fungal pathogens.
- Hematology: Controls for blood cell counts and coagulation tests.
- By End-User:
- Hospitals and Clinics: Major users of IVD quality control products for routine testing.
- Diagnostic Laboratories: Specialized labs focusing on high-volume and specialized testing.
- Research Institutes: Users of quality control for research and development purposes.
- Point-of-Care Testing Centers: Facilities providing rapid testing in various settings.
- By Region:
- North America: Dominates the market due to advanced healthcare infrastructure and high adoption of IVD technologies.
- Europe: Significant market share owing to stringent regulatory standards and growing demand for diagnostic tests.
- Asia-Pacific: Fastest-growing region, driven by increasing healthcare investments and rising awareness of diagnostic testing.
- Latin America and Middle East Africa: Emerging markets with potential for growth due to improving healthcare facilities.
Key Players
Several companies are leading the IVD quality control market, including:
- Bio-Rad Laboratories, Inc.
- Thermo Fisher Scientific Inc.
- Roche Diagnostics
- Siemens Healthineers
- Abbott Laboratories
- Randox Laboratories Ltd.
- Quidel Corporation
Future Outlook
The IVD quality control market is expected to continue its upward trajectory, driven by ongoing technological advancements, increasing healthcare expenditure, and a growing emphasis on accurate and reliable diagnostics. Innovations in digital health and artificial intelligence are likely to further enhance quality control processes, providing new opportunities for market growth.
Conclusion
The IVD quality control market is a critical component of the healthcare industry, ensuring the reliability and accuracy of diagnostic tests. With continuous advancements and a growing focus on quality in diagnostics, the market is poised for sustained growth and innovation.
Get 10% Free Customization on this Report: https://www.marketsandmarkets.com/requestCustomizationNew.asp?id=198032582
Exploring the IVD Quality Control Market ?
The In Vitro Diagnostics (IVD) Quality Control Market is experiencing significant growth and transformation. As healthcare technologies advance, the importance of maintaining high standards in diagnostic testing has never been more crucial.
Here are some key insights:
- Market Growth: The IVD quality control market is expanding rapidly, driven by the increasing prevalence of chronic diseases, growing awareness about the importance of early diagnosis, and advancements in laboratory automation.
- Technological Advancements: Innovations in quality control products are enhancing the accuracy and reliability of diagnostic tests. AI and machine learning are playing pivotal roles in developing more efficient and precise quality control systems.
- Regulatory Landscape: Stricter regulations and standards are being implemented globally, ensuring that diagnostic tests meet the highest quality benchmarks. This is driving the demand for comprehensive quality control solutions.
- Key Players: Leading companies in the market are continuously investing in RD to offer advanced quality control solutions. Collaboration and partnerships are also on the rise, fostering innovation and improving market reach.
- Future Prospects: With continuous technological advancements and increasing healthcare investments, the IVD quality control market is poised for substantial growth, promising better diagnostic accuracy and improved patient outcomes.